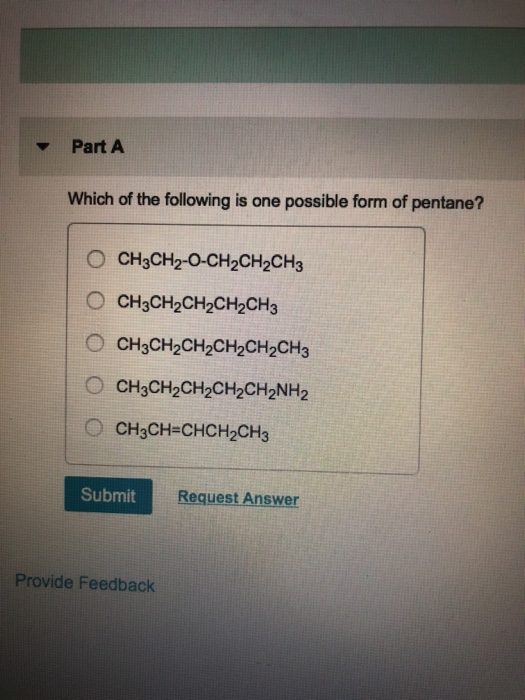
Which of the Following Is One Possible Form of Pentane
Up to 256 cash back 1-bromo-14-dimethylcyclohexane b. The mere exposure effect provides one possible explanation for why _____ increases attraction.
R S Pentane 2 4 Diol C5h12o2 Pubchem
The total number of stereoisomers of the compound.
. C H 2 SO 3. How many monpocarboxylic acids are possible which one decarboxylation from iso - pentane. Pentane has three structural isomers that are n-pentane Iso-pentane methyl butane and neopentane dimethylpropane.
Isomer 2 is 2 - methylbutane a branched chain with a carbon atom joined onto three other carbon atoms. Which of the following is not one of the possible consumer reference prices. C CH 3 CH 2 CH 2 CH 2 CH 2 CH 3.
Neo -Pentane or 2 2 -dimethylpropane has only primary hydrogen hence it can give only monochloro derivative. A CH 3 CH 2 CH 2 CH 2 CH 3. Isobutane is a structural isomer of butaneButane is unbranched and isobutane is branched.
Note that for all the isomers. How many isomers are possible for pentene. 27 Which of the following is one possible form of pentane.
Which of the following is one possible form of pentane. Therefore three structural isomers can be drawn from pentane. Thus neo-pentane is correct answer.
Answer is CH3CH2CH2CH2CH3 Explanation- Pentane belongs to alkane family which has gene. 52 Which of the following is one possible form of pentane. There are three isomers of pentane C5H12 which are shown in the following image.
All other compounds have 1 2 and 3 hydrogens hence they give mono di or trisubstituted products. Cyclopentane is not an isomer of pentane because it has only 10 hydrogen atoms where pentane has 12. There are five isomers of pentene.
Select the true statement about benzene amongst the following. The total number of aldehydes and ketones with the molecular formula C 4 H 8 O is - A 2 B 3 C 4 D 5 2 3. Since neo-pentane has only primary hydrogens thus it can give only monochloro derivative.
A because of unsaturation benzene easily undergoes addition. C there is cyclic delocalisation of p-electrons in benzene. Cyclopentane also called C pentane is a highly flammable alicyclic hydrocarbon with chemical formula C5H10 and CAS number 287-92-3 consisting of a ring of five carbon atoms each bonded with two hydrogen atoms above and below the plane.
Are butane and isobutane structural isomers. The structures of isomers of Pentane are. Which of the following would be expected to form hydrogen bonds with water.
B there are two types of C C bonds in benzene molecule. 1-bromopentane 2-bromopentane and 3-bromopentane. All other compounds have 1 2 and 3 hydrogens hence they give mono di or trisubstituted products.
Depending on the number of types of Hydrogen present the chain isomers on photochemical chlorination will yield single or multiple monochlorinated positional isomers -. How many meso stereoisomers are possible for 2 3 4-pentanetriol - A 1 B 2 C 3 D none of these 2 1. The reason there are only three derivatives is because of resonance.
How is mono-carboxylic acid prepared from ester. Get the detailed answer. Isomer 3 is 2 2 - dimethylpropane a branched chain with the central carbon atom joined onto four other carbon atoms.
N-pentane IUPAC name- Pentane 2 Common name- iso pentane IUPAC name- 2-methyl butane. In the IUPAC nomenclature however pentane means exclusively the n -pentane isomer. CH2CHCHCH2CH3 O CH3CH2CH2CH2CH3 O CH3CH2-O-CH2CH2CH3 CH3CH2CH2CH2CH2NH2 CH3CH2CH2CH2CH2CH3.
D monosubstitution of benzene gives three isomeric products. Pentene isomers contain one double bond between the two carbon apart from 5 carbon and 10 hydrogen atoms. D CH 3 CH 2 CH 2 CH 2 CH 2 NH 2.
B Isopentane has four types of hydrogen that on. Which one of the following event is possible in wireless lan. E CH 3 CH 2 - O - CH 2 CH 2 CH 3.
A CH3CH2CH2CH2CH2CH3 B CH3CH2CH2CH2CH2NH2 C CH3CH2CH2CH2CH3 D CH3CHCHCH2CH3 E CH3CH2-O-CH2CH2CH3 27 28 Which of the following compounds is ethanol. View the full answer. Pentane C 5 H 12 is an organic compound with five carbon atomsPentane has three structural isomers that are n-pentane Iso-pentane methyl butane and neopentane dimethylpropane.
On the left is pentane or 1-pentane which is sometimes called n-pentane. It occurs as a colorless liquid with a petrol-like odor. Answer 1 of 9.
In which of the following properties do enantiomers differ from. Kaidyn Answeregy Expert How many different monochlorinated compounds produced by. The other two are called isopentane methylbutane and neopentane dimethylpropane.
Correct option is A Answer- A neo-Pentane. Pentane C 5 H 12 is an organic compound with five carbon atoms. 1-pentene 2-pentene 2-methyl but-1-ene 3-methyl but-1-ene 2-methyl but-2-ene.
A C2H6 B CH3CO2CH3 C CH3CO2H D C2H5OH E CH3-O-CH3 28 29 Calculate the molar mass for MgClO42. In the middle is 2-methylbutane but its common name is isopentane. Learn vocabulary terms and more with flashcards games and other study tools.
Hence there are 5 structural isomers of pentene as mentioned above. What are the names of all monobromo derivatives of pentane. Which of the following reagents is used for.
Pentane has three different monobromo derivatives possible. Isomer 1 is n - pentane the straight chain normal structure for pentane. A form created from more than one table uses the primary table for the main form.
Outline all steps in a possible laboratory synthesis of each of the following compounds from benzene toluene and alcohols of four. A n-pentane has three types of hydrogen that on monochlorination gives 1-chloropentane 2-chloropentane and 3-chloropentane. The term may refer to any of three structural isomers or to a mixture of them.
B CH 3 CH CHCH 2 CH 3. How many monpocarboxylic acids are possible which one decarboxylation from iso - pentane.

Pentane Hplc Fisher Chemical Fisher Scientific

Pentane Reagent Cas 109 66 0 P1013 Spectrum Chemical

Pentane An Overview Sciencedirect Topics

Tad Toulis Pentane Machine Design Portfolio Design Technology Design

1 5 Pentanediol C5h12o2 Pubchem

Propanal Propanone And Pentane 3 One Pentane 2 One Represent The Isomerism Of Which Type Respectively

Organocuprates Gilman Reagents How They Re Made Organic Chemistry Organic Chem Chemistry
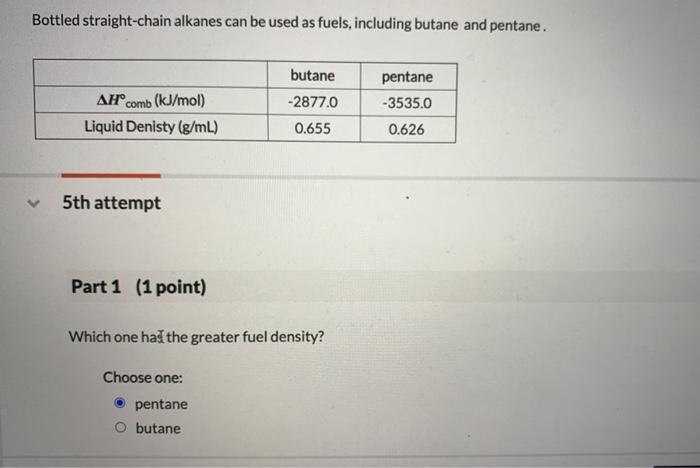
Solved Bottled Straight Chain Alkanes Can Be Used As Fuels Chegg Com

Chain Isomerism Vs Position Isomerism Tabular Form Functional Group Positivity Chemical Formula

Chemical Reactions Chemical Reactions Chemical Flow Chart

Pentane Machine Design Geometry Design Design Skills
Solved Part A Which Of The Following Is One Possible Form Of Chegg Com

How Many Structure Isomers Can You Draw For Pentane 1 2 2 4 3 3 4 6 Chemistry Q A

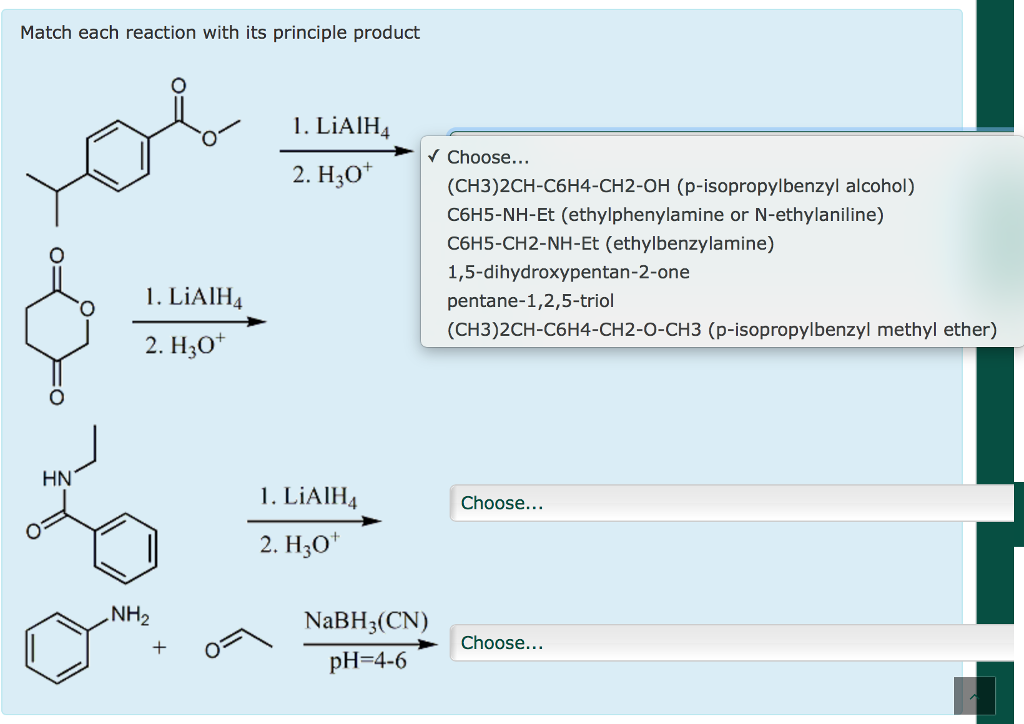
Solved Match Each Reaction With Its Principle Product Chegg Com
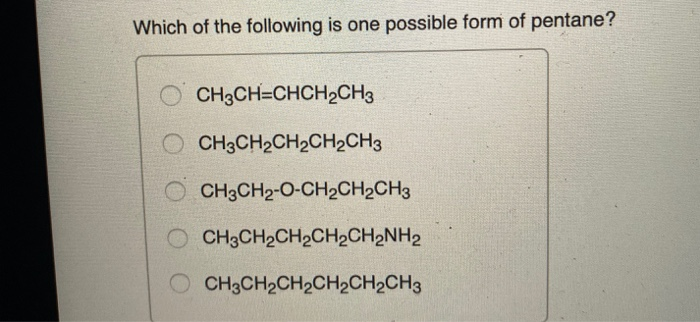
Solved Which Of The Following Is One Possible Form Of Chegg Com
Chemicals And Solvents Lab Pro Inc
Comments
Post a Comment